Creating L-Arginine tablets involves several steps, from ingredient selection to tablet formulation and manufacturing. Below is a general outline of the materials and methods involved:
Materials:
1.Active Ingredient:
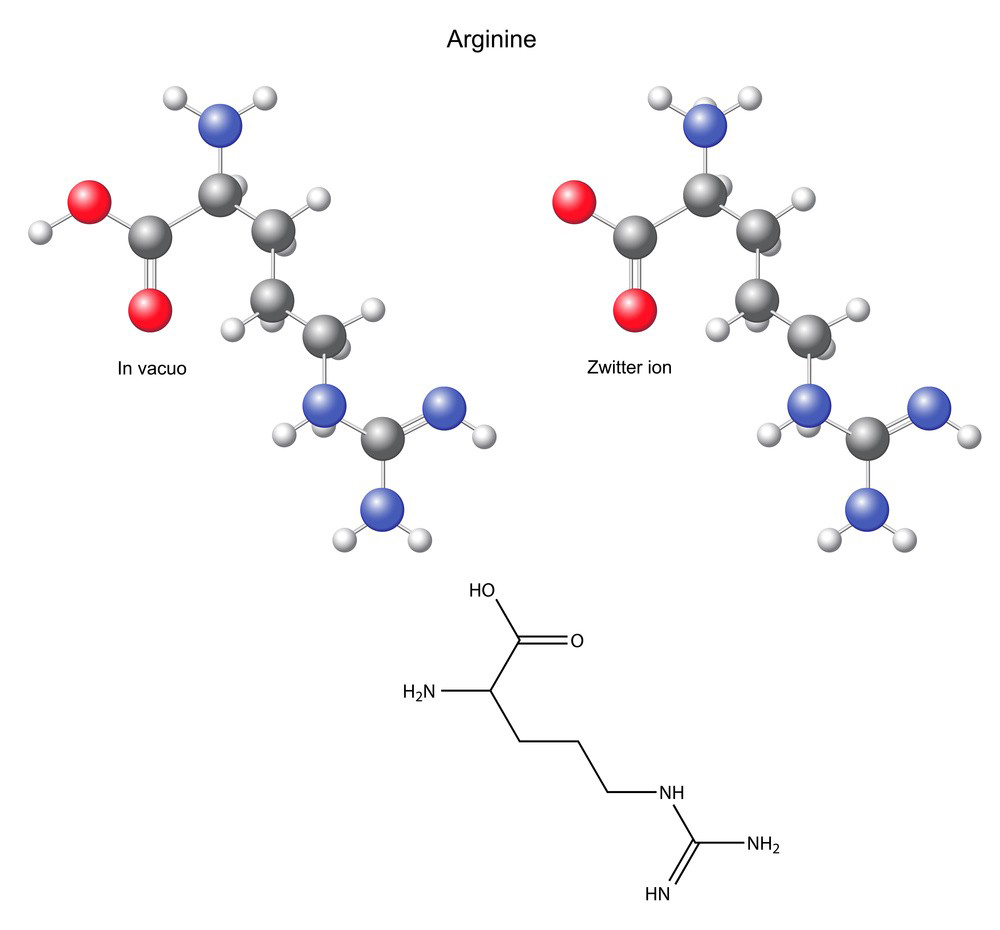
L-Arginine: Obtain pharmaceutical-grade L-arginine powder.
2.Excipients:
Binder: To bind the powder particles together. Common binders include microcrystalline cellulose, starches, or polyvinylpyrrolidone (PVP).
Disintegrant: To facilitate the breakup of the tablet upon ingestion. Examples include croscarmellose sodium or sodium starch glycolate.
Lubricant: To prevent tablet sticking to punches and dies during compression. Examples include magnesium stearate or stearic acid.
Filler: To add bulk to the tablet. Common fillers include lactose, calcium phosphate, or mannitol.
Glidant (optional): To improve flow properties of the powder blend. Examples include colloidal silicon dioxide or talc.
3.Equipment:
Mixing equipment (blender or mixer)
Tablet compression machine
Tablet coating machine (if film-coated tablets are desired)
Sieves (if necessary for particle size reduction)
Drying equipment (if necessary)
Methods:
1.Weighing and Mixing:
Weigh the required quantities of L-Arginine (active ingredient) and excipients according to the formulation.
Mix the ingredients thoroughly in a blender or mixer to ensure uniform distribution.
2.Granulation (optional):
Granulation may be performed to improve flow properties and tablet uniformity. It involves wet or dry granulation techniques.
3.Compression:
After mixing or granulation, the blend is compressed into tablets using a tablet compression machine.
Adjust the compression force to achieve the desired tablet hardness.
4.Coating (optional):
If desired, tablets can be coated to improve appearance, taste masking, or to provide enteric properties.
Film coating can be applied using a tablet coating machine.
5.Drying (if necessary):
If wet granulation or coating is performed, drying may be required to remove moisture.
6.Quality Control:
Conduct quality control tests such as weight variation, hardness, disintegration, and assay to ensure the tablets meet the required standards.
7.Packaging:
Pack the tablets into suitable packaging materials, ensuring proper labeling with dosage instructions, batch number, expiry date, etc.
8.Storage:
Store the finished tablets in appropriate conditions to maintain their stability until distribution.
It’s important to note that specific formulations and manufacturing processes may vary depending on factors such as dosage strength, tablet size, desired release profile, and regulatory requirements. Therefore, it’s advisable to consult pharmaceutical formulation experts and adhere to Good Manufacturing Practices (GMP) guidelines throughout the manufacturing process.
