Chitosan is a biopolymer derived from chitin, a natural polymer found in the exoskeletons of arthropods like shrimp, crabs, and insects. The process of synthesizing chitosan typically involves the deacetylation of chitin. Here’s a general outline of the synthesis method:
1. Extraction of Chitin:
- Source: Chitin is usually extracted from the shells of crustaceans (shrimp, crab) or insects.
- Process: The shells are cleaned to remove impurities such as proteins, lipids, and minerals. This is typically done using a combination of washing, soaking, and mechanical processes.

2. Deacetylation of Chitin to Chitosan:
- Chemical Process: Chitin is deacetylated by treatment with a strong alkaline solution, typically sodium hydroxide (NaOH), at high temperatures. This removes the acetyl groups (-COCH3) from the chitin molecule, resulting in chitosan.
- Reaction:
Chitin+NaOH→Chitosan+Acetate Ion
- Conditions: The reaction usually occurs at temperatures ranging from 60°C to 120°C, and the degree of deacetylation (DD) depends on the concentration of NaOH and the reaction time.
3. Purification:
- After deacetylation, chitosan is often purified by washing with water and possibly dilute acid to neutralize any remaining alkali. The purified chitosan is then dried.
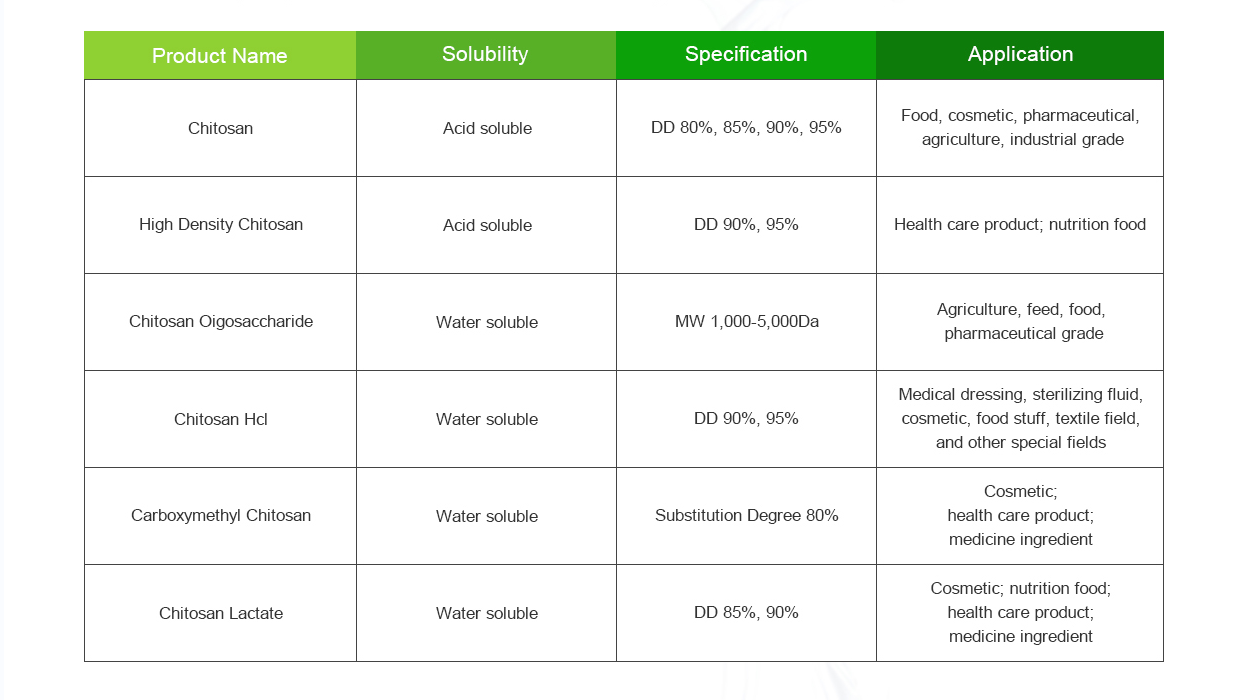
4. Characterization:
- The degree of deacetylation (DD) is a critical factor that determines the solubility and other properties of chitosan. DD can be measured using techniques like Fourier-transform infrared spectroscopy (FTIR) or nuclear magnetic resonance (NMR) spectroscopy.
5. Optional Modifications:
- Modification for Specific Applications: Chitosan can be modified further to improve its properties, such as increasing its solubility in acidic solutions, enhancing its bioactivity, or making it more hydrophobic for specific applications (e.g., in drug delivery, food preservation, or wound healing).
This method produces chitosan, which has a wide range of applications due to its biocompatibility, biodegradability, and antimicrobial properties.
