Minoxidil, a medication primarily used to treat hair loss, has a fascinating history that began with its development as a treatment for high blood pressure. Here’s a brief overview of its history:
1. Development and Discovery (1950s–1970s)
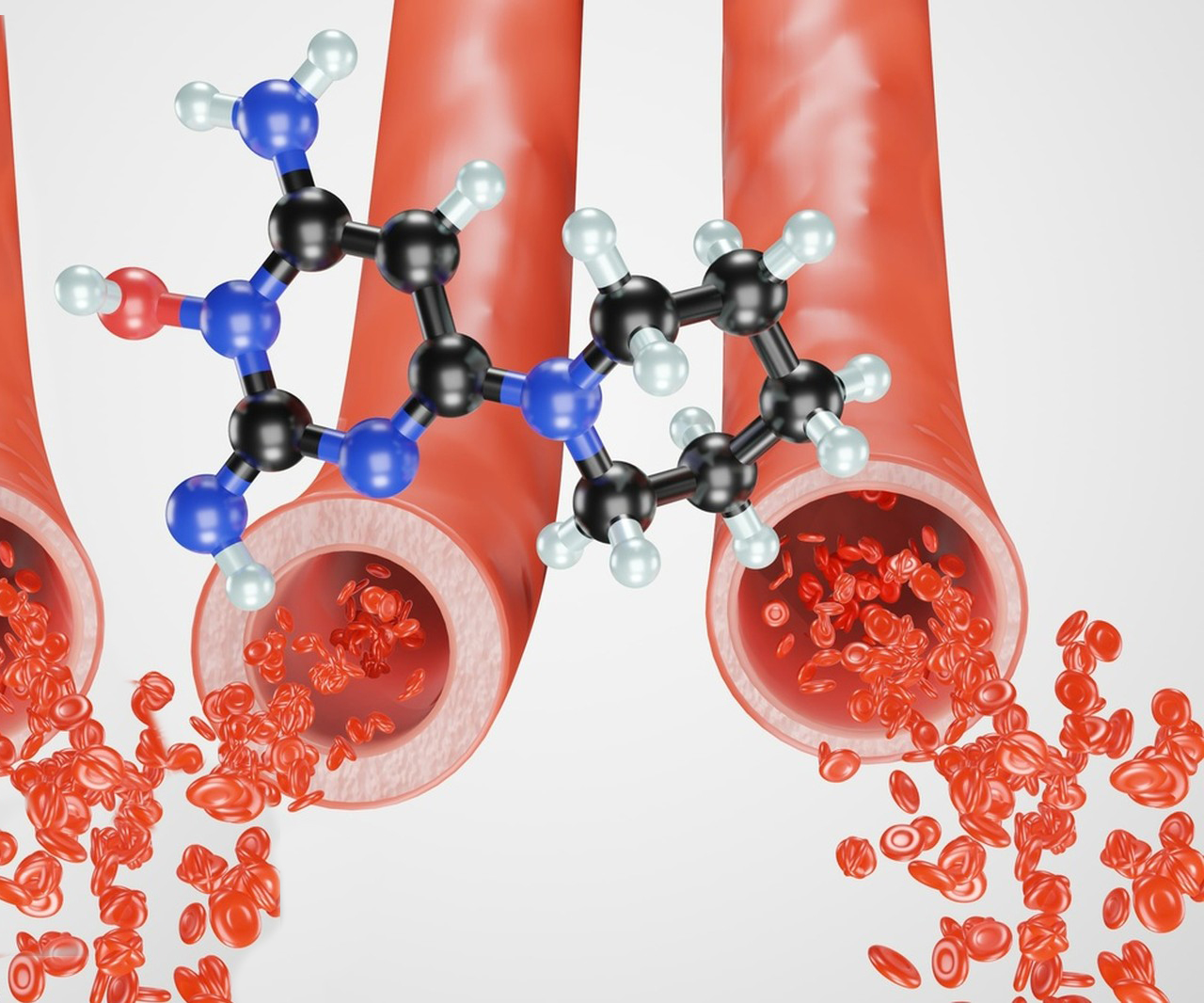
Minoxidil was initially developed by the pharmaceutical company Upjohn (now part of Pfizer) in the 1950s and 1960s as part of their research into antihypertensive medications, or drugs to lower blood pressure. It was originally known by its chemical name, 2,4-diamino-6-piperidinopyrimidine-3-oxide, and was first formulated as an oral medication for severe hypertension. In 1979, the U.S. FDA approved it for use under the brand name Loniten.
2. Unexpected Side Effect (Late 1970s–1980s)
While Minoxidil was effective at lowering blood pressure, an unexpected side effect of the drug was excessive hair growth. Patients taking the oral medication began reporting new hair growth on their faces and bodies, a phenomenon known as hypertrichosis. This surprising effect led to investigations into whether Minoxidil could be used for treating hair loss.

3. Development of Topical Formulation (1980s)
In response to the growing interest in Minoxidil’s ability to promote hair regrowth, researchers began developing a topical version of the drug, which could be applied directly to the scalp to treat androgenetic alopecia (commonly known as male-pattern baldness and female-pattern baldness). After clinical trials, the topical form of Minoxidil was approved by the U.S. Food and Drug Administration (FDA) in 1988 for over-the-counter use, marketed under the brand name Rogaine.
4. Expanded Use and Market Success (1990s–2000s)
The introduction of Rogaine to the market was a breakthrough in the treatment of hair loss. Minoxidil became one of the first FDA-approved treatments for pattern baldness, and its popularity surged during the 1990s. In the following years, Minoxidil became available in different formulations, including foam and liquid versions, and at various concentrations (e.g., 2% and 5%).
The drug’s ability to stimulate hair growth was attributed to its vasodilation (widening of blood vessels) properties, which increase blood flow to hair follicles, encouraging their growth and health.
5. Evolving Understanding and Continued Use (2010s–Present)
Minoxidil continues to be a mainstay in the treatment of hair loss, particularly androgenetic alopecia. It is used by millions of people worldwide, both as a treatment for male and female pattern baldness. Though its precise mechanism of action is still not fully understood, it is believed to not only promote hair regrowth but also to prolong the anagen (growth) phase of the hair cycle.
In addition to Rogaine, generic versions of Minoxidil and newer branded products have entered the market, increasing the availability and affordability of the drug.
6. Ongoing Research
While Minoxidil is effective for many people in slowing hair loss and promoting regrowth, it does not work for everyone. Ongoing research into better formulations and combination therapies continues, with some studies looking into the potential for combined treatments that may enhance Minoxidil’s effectiveness, such as the use of finasteride (another FDA-approved treatment for hair loss) alongside Minoxidil.
Summary of Key Milestones:
- 1950s–1960s: Minoxidil developed as an antihypertensive medication.
- 1979: Approved for use in hypertension under the brand name Loniten.
- 1980s: Discovery of hair growth side effect leads to development of topical form.
- 1988: FDA approval of topical Minoxidil (Rogaine) for hair loss.
- 1990s: Minoxidil becomes a widely used treatment for androgenetic alopecia.
- Present Day: Minoxidil remains a leading over-the-counter solution for hair regrowth.
While Minoxidil is still one of the most common treatments for hair loss, its exact mechanism of action and long-term efficacy continue to be subjects of scientific study.
