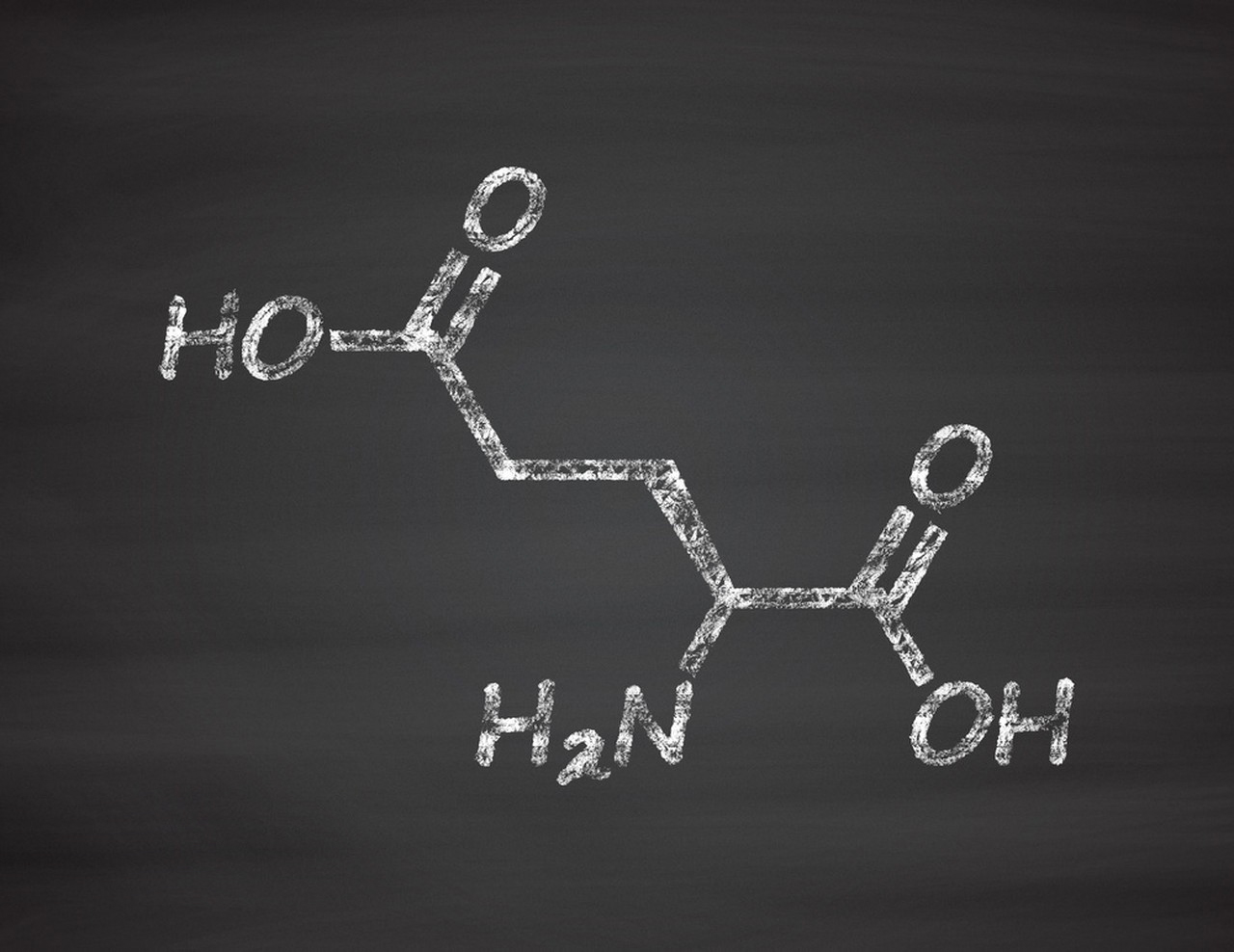
L-Glutamic acid is a non-essential amino acid that plays a crucial role in various biochemical processes in the human body. It is also used in the food and pharmaceutical industries. The processing of L-Glutamic acid can involve several steps, depending on the source and intended use. Here is an overview of the typical processing process:
1.Fermentation or Extraction: L-Glutamic acid can be produced through two primary methods: fermentation and extraction.
- Fermentation: This is the most common method. Bacteria, such as Corynebacterium glutamicum, are used to ferment carbohydrates, like glucose or molasses, in a bioreactor. These bacteria naturally produce L-Glutamic acid as part of their metabolic processes. The fermentation process is carefully controlled to optimize the yield of L-Glutamic acid.
- Extraction: In some cases, L-Glutamic acid can also be extracted from natural protein sources like wheat gluten or soy protein. This method involves breaking down the proteins and then isolating and purifying the amino acid.
2.Filtration and Separation: After the fermentation or extraction process, the mixture containing L-Glutamic acid needs to be filtered and separated from the remaining components, such as cells, proteins, and other impurities. This is typically done through centrifugation and filtration processes.
3.Concentration: The L-Glutamic acid solution is then concentrated to increase its purity and potency. This is often done by evaporating excess water, leaving behind a more concentrated solution of L-Glutamic acid.
4.Crystallization: To further purify L-Glutamic acid, it can be crystallized. Crystallization involves cooling the concentrated solution, allowing the amino acid to form crystals. These crystals are separated from the remaining liquid.
5.Drying: The isolated L-Glutamic acid crystals are then dried to remove any remaining moisture. This results in a dry, powdered form of L-Glutamic acid.
6.Quality Control: Throughout the processing steps, quality control measures are implemented to ensure the purity and safety of the L-Glutamic acid product. This includes testing for impurities, microbial contamination, and other quality parameters.
7.Packaging: The final product is packaged into containers suitable for its intended use. In the food industry, L-Glutamic acid may be sold as a food additive, often as monosodium glutamate (MSG). In the pharmaceutical industry, it can be used as a component in drug formulations.
It’s important to note that the specific processing steps and conditions can vary depending on the manufacturer and the intended application of L-Glutamic acid. Strict quality control and regulatory compliance are essential to ensure the safety and quality of the final product, especially when it is used in food or pharmaceuticals.
