Gentian violet are closely related compounds, and they are both synthetic dyes with similar chemical structures. Here is some information about their chemical structure and physical properties:
Chemical Structure of Gentian violet:
1.Crystal Violet:
Chemical Name: Triarylmethane dye
Chemical Formula: C25H30N3Cl
Structure: Crystal violet is a triphenylmethane dye, meaning it has three phenyl (aromatic) rings attached to a central carbon atom.
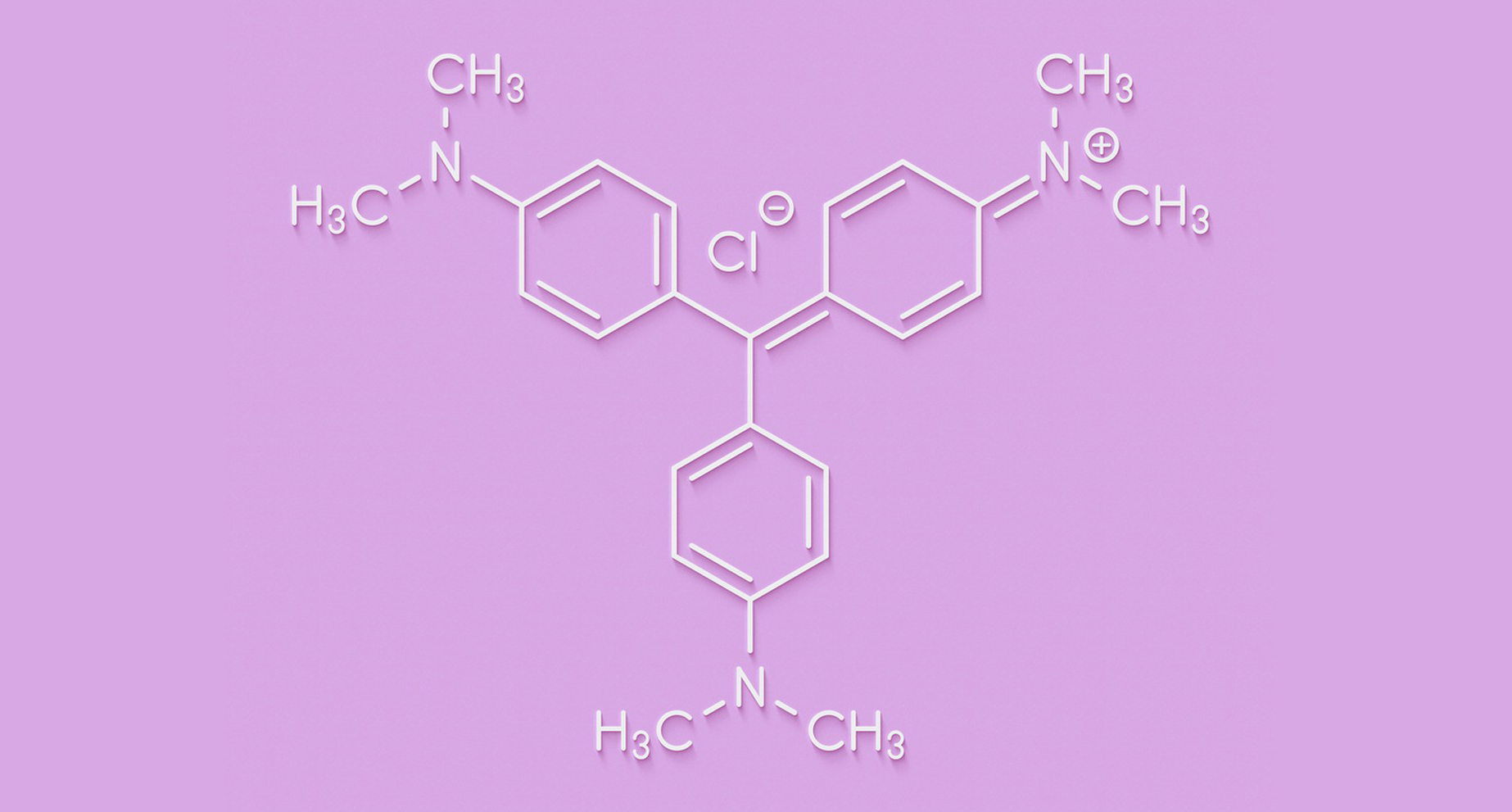
Chemical Name: Methylrosaniline chloride
Chemical Formula: C25H30ClN3
Structure: Gentian violet is also a triphenylmethane dye, similar to crystal violet. It has three phenyl rings and a central carbon atom.
Physical Properties of Gentian violet:
1.Color:
Both gentian violet are known for their deep violet or purple color.
2.Solubility:
Crystal violet and gentian violet are typically soluble in water, which makes them suitable for use as water-soluble dyes.
3.Applications:
These dyes are commonly used in histology and microbiology for staining tissues and cells.
4.Biological Staining:
Gentian violet are often used as biological stains, especially in Gram staining procedures to differentiate between Gram-positive and Gram-negative bacteria.
5.Antifungal Properties:
Gentian violet, in particular, has antifungal properties and has been used in the past as a topical treatment for fungal infections.
6.Photophysical Properties:
Both dyes exhibit fluorescence under certain conditions, and this property has been utilized in various applications, including fluorescence microscopy.
It’s important to note that while these dyes have similar structures and properties, there may be slight differences in their specific applications and behaviors. Additionally, the use of gentian violet in medical applications has diminished over time due to safety concerns, and alternatives are often preferred.
