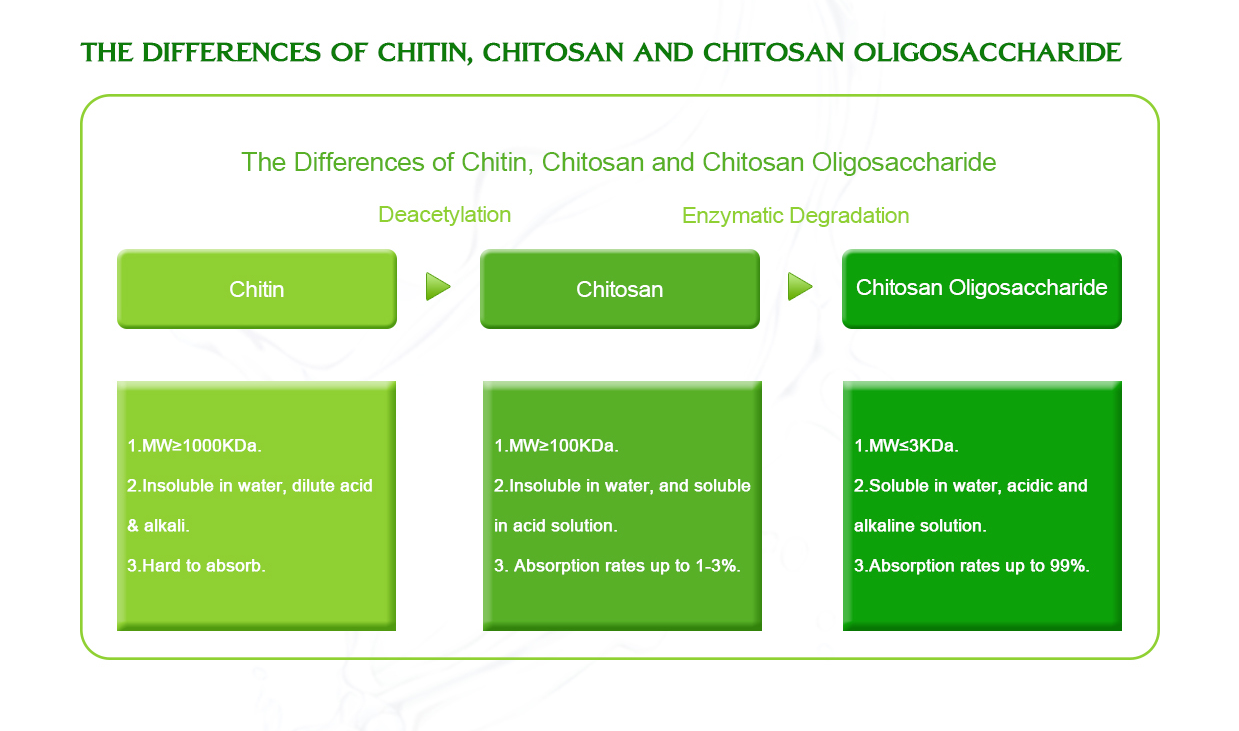
Chitosan is a biopolymer derived from chitin, primarily found in the exoskeletons of crustaceans like shrimp and crabs. It is often used in various applications, such as medicine, food preservation, and water treatment, due to its biodegradable, biocompatible, and non-toxic nature. The preparation of chitosan typically involves the deacetylation of chitin using either an acidic or alkaline process. Here’s a brief explanation of the acid and alkali methods for preparing chitosan:
1. Alkaline Preparation of Chitosan (Deacetylation)
The alkaline method is the most commonly used process for the preparation of chitosan from chitin. The process involves the deacetylation of chitin, which is the removal of acetyl groups (-COCH3) from the chitin structure to form chitosan.
Steps:
- Chitin Source: The chitin is usually obtained from shrimp shells, crab shells, or other crustacean exoskeletons.
- Alkaline Hydrolysis: Chitin is treated with a strong alkali, commonly sodium hydroxide (NaOH), in concentrations ranging from 40–50% at elevated temperatures (often 80–100°C) for several hours. This breaks the acetyl group from the chitin molecule and converts it to chitosan.
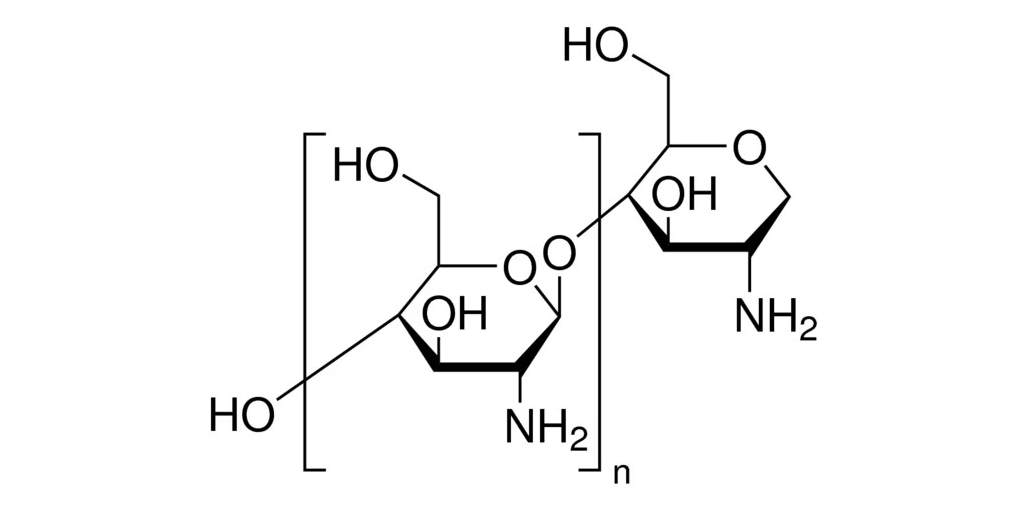
- Chemical Reaction: Chitin+NaOH→Chitosan+Acetic acid
- Purification: After the deacetylation reaction, the chitosan is typically purified by washing with distilled water to remove residual alkali and other impurities.
- Drying: The purified chitosan is then dried, often by air-drying or freeze-drying, to obtain the final product in powder form.
Advantages of Alkali Preparation:
- Simple and widely used method.
- High degree of deacetylation, yielding a higher molecular weight chitosan.
- Effective for large-scale production.
2. Acidic Preparation of Chitosan
The acidic method of preparing chitosan is less common and generally involves the initial treatment of chitin with a strong acid to remove proteins and minerals before deacetylation with alkali. This method is typically used when a purer form of chitin is needed before undergoing the deacetylation step.
Steps:
- Deproteinization: Chitin is first treated with a strong acid like hydrochloric acid (HCl) or sulfuric acid (H2SO4) to remove proteins and other contaminants from the chitin.
- Deacetylation: After deproteinization, the chitin is then treated with an alkali (e.g., NaOH) for deacetylation, similar to the alkaline method above.
The acidic step is often used to ensure that the chitin is as clean as possible, which can lead to higher-quality chitosan after deacetylation.

Chemical Reaction (Overall Process):
- Deproteinization (using acid): Chitin+HCl→Deproteinized Chitin+Protein Hydrolyzates
- Deacetylation (using alkali): Deproteinized Chitin+NaOH→Chitosan+Acetic Acid
Comparison of Acid and Alkali Methods
Alkaline Method:
- Directly deacetylates chitin.
- Requires high concentrations of NaOH.
- Often produces chitosan with a higher molecular weight.
- More common in industrial applications.
Acidic Method:
- Often used in combination with alkali treatment to purify chitin before deacetylation.
- Produces cleaner chitosan but is more time-consuming.
- Can lead to lower molecular weight chitosan if care is not taken.
Factors Affecting Chitosan Preparation:
- Deacetylation Degree: The extent to which the acetyl group is removed from the chitin molecule determines the degree of deacetylation, which affects the solubility and functional properties of the resulting chitosan.
- Reaction Time and Temperature: These factors can influence the molecular weight and viscosity of the chitosan.
- Concentration of Reagents: The concentration of sodium hydroxide or hydrochloric acid used during the process can affect the yield and quality of the chitosan.
Would you like more specific details on one of these steps or the applications of chitosan?
