Dimercaptosuccinic acid, commonly known as DMSA, is a chemical compound used in medicine as a chelating agent. It is often administered to treat heavy metal poisoning, particularly lead poisoning, by facilitating the excretion of these metals from the body. Here is some information about the chemical structure and physical properties of DMSA:
Chemical Structure of DMSA:
DMSA’s chemical structure is as follows:
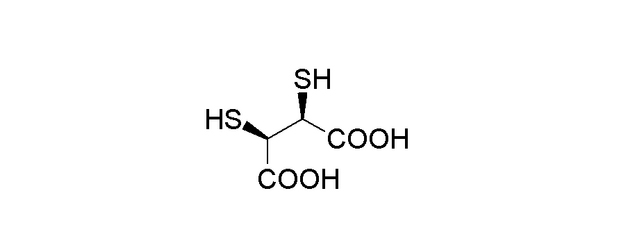
It has two thiol (–SH) groups and two carboxylic acid (–COOH) groups. The thiol groups are important for chelation as they can bind to heavy metal ions.
Physical Properties of DMSA:
- Molecular Weight: The molecular weight of DMSA is approximately 182.2 g/mol.
- Appearance: DMSA is typically a white crystalline solid.
- Solubility: It is soluble in water and forms clear solutions. The solubility of DMSA may vary with temperature and pH.
- Melting Point: DMSA’s melting point is around 190-192°C (374-378°F).
- pKa Values: The pKa values of the carboxylic acid groups in DMSA are around 2.95 and 4.51. This means that at physiological pH (around 7.4), these groups will be largely ionized.
Chelation Mechanism:
DMSA works as a chelating agent by forming coordinate bonds between its thiol groups and heavy metal ions, creating stable complexes. These complexes are water-soluble and can be excreted from the body through urine. The use of DMSA is a common approach in treating heavy metal poisoning, as it can help reduce the toxic effects of these metals.
It’s important to note that DMSA is used under medical supervision, and its dosing and administration should be determined by a qualified healthcare professional based on the specific condition being treated.
Please be aware that the information provided here is based on my knowledge up to September 2021, and there may have been developments or new findings since then.
