L-Glutamic acid, often simply referred to as glutamic acid, is one of the 20 standard amino acids that make up proteins in living organisms. Here’s some information about its chemical structure and physical properties:
Chemical Structure of L-Glutamic Acid:
- Molecular Formula: C5H9NO4
- Systematic Name: 2-Aminopentanedioic acid
- Structural Formula:
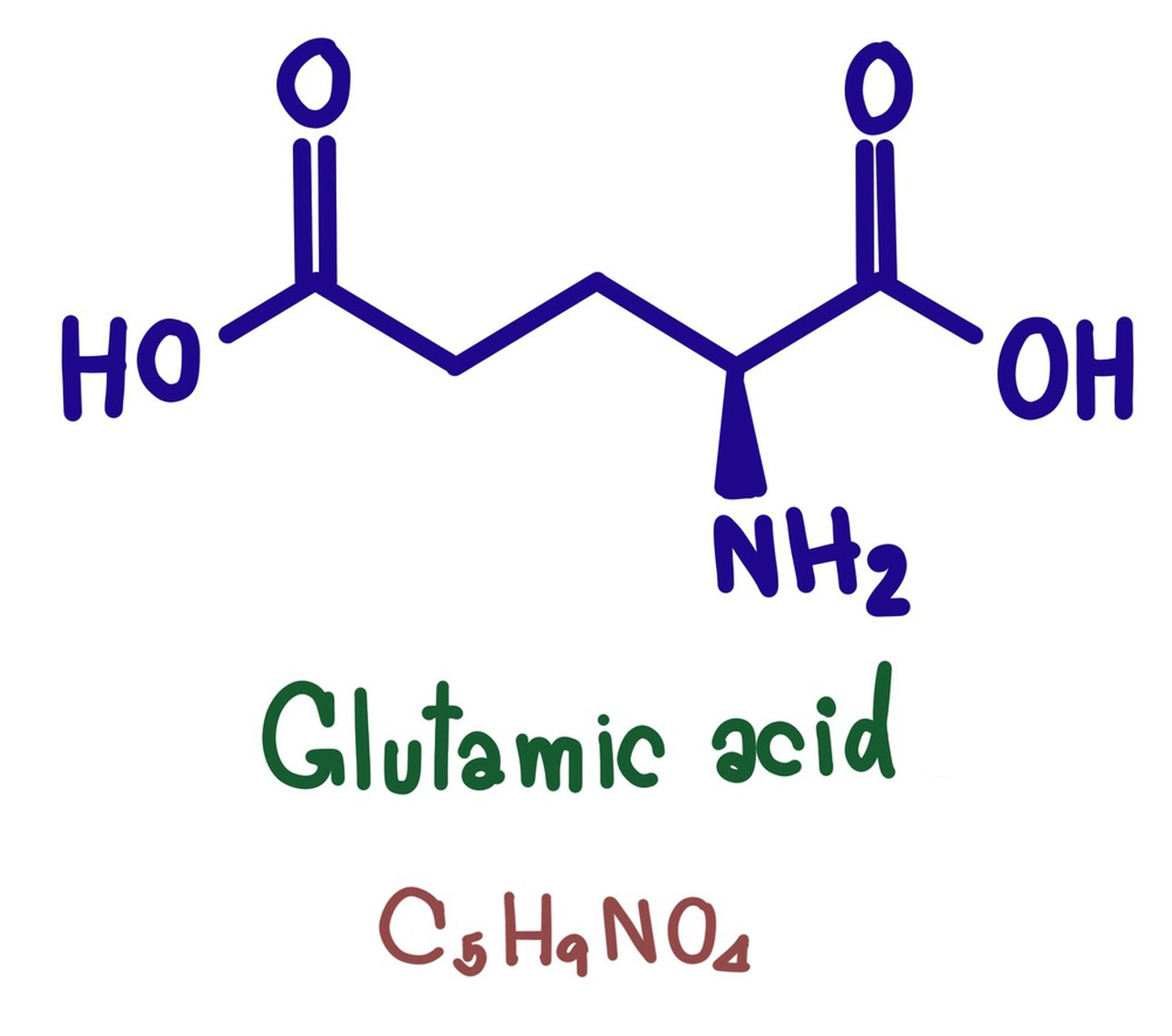
- L-Glutamic acid is an α-amino acid, which means that the amino group (-NH2) is attached to the carbon atom adjacent to the carboxyl group (-COOH) in the molecular structure.
Physical Properties of L-Glutamic Acid:
- Appearance: Glutamic acid is a white, odorless, and crystalline solid at room temperature.
- Solubility: It is highly soluble in water but insoluble in organic solvents like ether, benzene, and chloroform.
- Melting Point: The melting point of L-glutamic acid is around 200-220 °C (392-428 °F).
- pKa Values: L-Glutamic acid has two ionizable groups: the carboxyl group (-COOH) and the amino group (-NH2). The pKa values for its ionization are approximately 2.19 and 4.25, respectively. This means that at low pH (acidic conditions), the carboxyl group is predominantly in its protonated form (-COOH), while at higher pH (basic conditions), the amino group becomes deprotonated (-NH3+ → -NH2).
- Taste: Glutamic acid is known for its umami taste, which is one of the five basic tastes alongside sweet, sour, bitter, and salty. Umami is often described as a savory or meaty taste and is associated with the presence of glutamate ions in foods.
- Hydrophobicity: L-Glutamic acid is a polar molecule due to the presence of the amino and carboxyl groups, making it hydrophilic (water-attracting).
- Biological Role: In addition to its role as a building block for proteins, glutamic acid is also a key neurotransmitter in the central nervous system, where it functions as an excitatory neurotransmitter. It is involved in various physiological processes, including learning, memory, and neural plasticity.
It’s important to note that there are two enantiomers of glutamic acid: L-glutamic acid and D-glutamic acid. L-glutamic acid is the naturally occurring form and is commonly found in proteins and food. D-glutamic acid, on the other hand, is less common in nature and is not used in protein synthesis.
L-glutamic acid and its salts, such as monosodium glutamate (MSG), are widely used as flavor enhancers in the food industry due to their ability to enhance the umami taste in foods.
