Niacin, also known as vitamin B3 or nicotinic acid, is an essential nutrient for human health and has both chemical and physical properties that are important to its function and effects in the body.
Chemical Structure of Niacin:
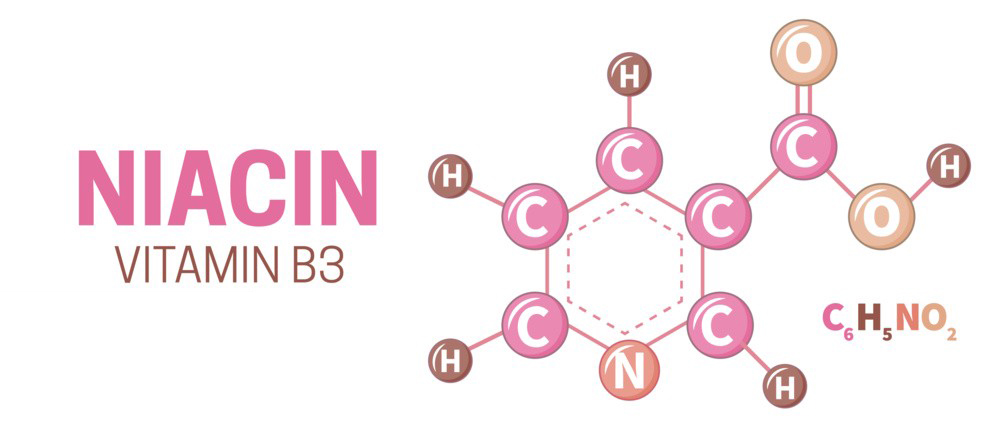
- Molecular Formula: C6H5NO2
- Structure: Niacin is a heterocyclic aromatic compound with a pyridine ring. It has a carboxylic acid group (–COOH) attached to the pyridine ring.
Physical Properties of Niacin:
- Appearance: Niacin is typically a white, crystalline solid.
- Solubility: It is soluble in water and alcohol but sparingly soluble in ether.
- Melting Point: The melting point of niacin is around 237-239°C.
- Odor: Niacin has a faint, characteristic odor.
Functions of Niacin:
- Vitamin Role: Niacin is essential for the synthesis of coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). These coenzymes play crucial roles in various metabolic reactions, including energy metabolism (such as glycolysis and the citric acid cycle) and cellular respiration.
- Health Benefits: Adequate niacin intake is important for overall health, including proper functioning of the digestive system, skin, and nerves. It also plays a role in lowering cholesterol levels.
Sources:
- Niacin is found naturally in many foods, including meat, fish, nuts, and grains. It can also be synthesized by the body from the amino acid tryptophan.

Deficiency and Toxicity:
- Deficiency: Severe niacin deficiency can lead to pellagra, a condition characterized by dermatitis, diarrhea, dementia, and eventually death if untreated.
- Toxicity: Excessive intake of niacin from supplements can cause niacin flush, which is characterized by flushing of the skin, itching, and tingling sensations. Very high doses can lead to liver damage.
In summary, niacin is a vital nutrient with a well-defined chemical structure and important physical properties that contribute to its role as a vitamin essential for human health and metabolism.
