Origin and Nature of DMSA:
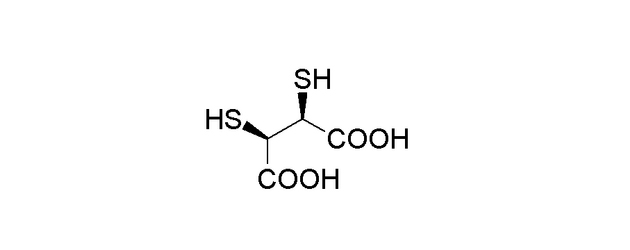
DMSA stands for Dimercaptosuccinic acid, a chelating agent that was first developed in the 1950s as part of efforts to treat heavy metal poisoning. Its molecular structure consists of two thiol (-SH) groups attached to a succinic acid backbone. The presence of these thiol groups allows DMSA to bind to and form stable complexes with metal ions, making it an effective agent for removing toxic metals like lead, mercury, and arsenic from the body.
Chemical Properties:
- DMSA is a water-soluble, organic compound.
- It is a chelating agent, meaning it binds to metals, forming a stable complex that can be excreted in the urine.
Introduction of DMSA:
DMSA was introduced primarily as an alternative to dimercaprol (BAL – British Anti-Lewisite), which had more severe side effects and required intramuscular injection. DMSA’s introduction in the medical field provided a safer, oral option for chelation therapy.
Historical Timeline:
- 1950s: Development of DMSA as a chelating agent for treating heavy metal poisoning.
- 1980s: Initial clinical studies began showing that DMSA was effective in treating lead poisoning, particularly in children.
- 1990s and beyond: DMSA gained popularity for treating lead and mercury toxicity, and it became an FDA-approved treatment for lead poisoning in children by 1998. It is also used in cases of other heavy metal intoxications, though it is often the first-line treatment for lead poisoning.
Mechanism of Action:
DMSA works by binding to toxic metals in the bloodstream and tissues. The thiol groups in DMSA form bonds with the metal ions, neutralizing their toxic effects. The resulting metal-DMSA complex is then excreted through the urine, effectively removing the heavy metal from the body. This process helps reduce the burden of metals that can accumulate in organs, especially the liver, kidneys, and brain.
Applications of DMSA:
- Lead Poisoning: DMSA is commonly used for treating lead toxicity, particularly in children, as lead can cause irreversible damage to the brain and nervous system.
- Mercury Poisoning: DMSA is used in cases of mercury exposure, particularly in individuals with chronic mercury intoxication.
- Arsenic Poisoning: DMSA can also be used to treat arsenic poisoning.
- Other Heavy Metal Intoxications: While primarily used for lead, mercury, and arsenic, DMSA may also be employed for other toxic metal exposures under medical supervision.
Advantages Over Other Chelating Agents:
- Oral Administration: Unlike other chelators, such as dimercaprol, which require injection, DMSA can be administered orally, making it easier for patients, especially children, to take.
- Lower Toxicity: DMSA is generally better tolerated compared to older chelating agents like dimercaprol, which can have significant side effects like hypertension, nausea, and kidney damage.
- Specificity for Lead: DMSA has a relatively high affinity for lead compared to other metals, which makes it an effective treatment for lead poisoning.

Side Effects of DMSA:
While DMSA is generally safer than many other chelating agents, it can cause some side effects, including:
- Gastrointestinal upset (nausea, vomiting, diarrhea)
- Skin rashes or allergic reactions
- Elevated liver enzymes (rare)
- Mild leukopenia (low white blood cell count)
Note: DMSA is used with caution in individuals with kidney disease or other underlying health issues, and it should always be administered under medical supervision, particularly in cases of heavy metal poisoning.
Conclusion:
DMSA represents a significant advancement in the treatment of heavy metal poisoning, offering a safer, effective, and more patient-friendly alternative to previous chelating agents. It remains one of the most commonly prescribed chelators for lead poisoning, especially in pediatric patients.
